A Smart Approach:
IMPEDE Embolization Plug Family
Generates new healing possibilities – Conforms to the anatomy – Returns clarity – Delivers unmatched volume
Success with Less
IMPEDE PRODUCT FAMILY
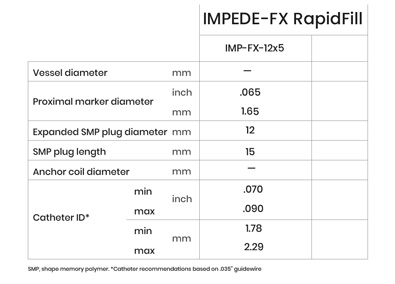
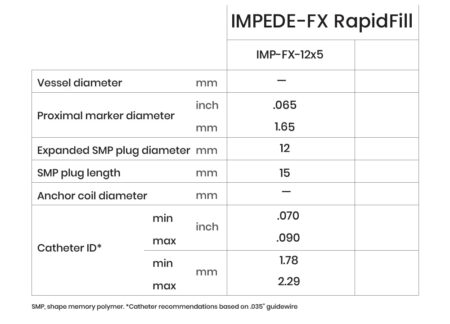
IMPEDE-FX RapidFill
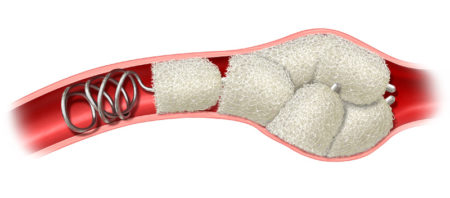
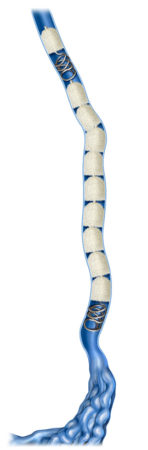
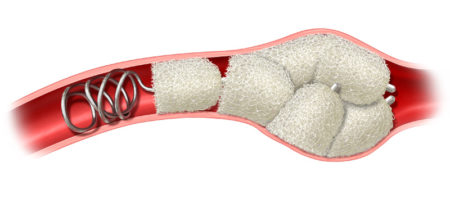
IMPEDE-FX RapidFill enables the delivery of 6.25 mL of smart polymer in a single application. The device consists of five IMPEDE-FX 12 mm Embolization Plugs preloaded into a single delivery introducer (cartridge), which may provide an advantage in cases where the goal is to quickly fill and occlude large vessels or lesions with reduced procedure and fluoroscopy time, contrast media, and blood loss.
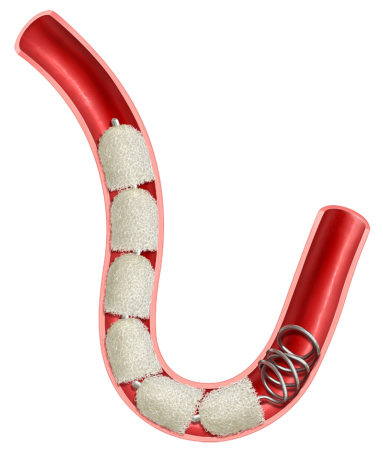
Space Fill with IMDEDE-FX RapidFill is a technique using multiple embolization plugs to fill and occlude large blood vessels or large spaces such as aneurysms or hemorrhage.
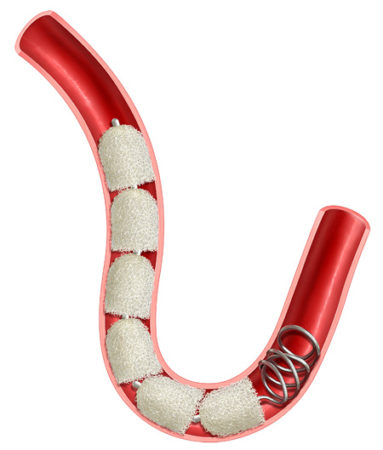
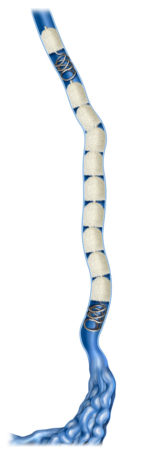
Track Fill with IMDEDE-FX RapidFill enables embolization along the vessel of lesion length, a technique as performed for venous insufficiency, pelvic congestion syndrome, and collateral vessel embolization.

INDICATIONS (Countries that recognize CE marking) – The IMPEDE-FX RapidFill Device is indicated to obstruct or reduce the rate of blood flow in the peripheral vasculature.
Market Approvals: The IMPEDE-FX RapidFill Device has received CE mark. IMPEDE-FX RapidFill is not available for sale in the United States or Japan. Find your local distributor.
Indications, contraindications, warnings, and instructions for use can be found in the product labeling supplied with each device. See all Indications, Safety & Warnings
IMPEDE PRODUCT FAMILY
IMPEDE-FX RapidFill
IMPEDE-FX RapidFill enables the delivery of 6.25 mL of smart polymer in a single application. The device consists of five IMPEDE-FX 12 mm Embolization Plugs preloaded into a single delivery introducer (cartridge), which may provide an advantage in cases where the goal is to quickly fill and occlude large vessels or lesions with reduced procedure and fluoroscopy time, contrast media, and blood loss.
Space Fill with IMDEDE-FX RapidFill is a technique using multiple embolization plugs to fill and occlude large blood vessels or large spaces such as aneurysms or hemorrhage.
Track Fill with IMDEDE-FX RapidFill enables embolization along the vessel of lesion length, a technique as performed for venous insufficiency, pelvic congestion syndrome, and collateral vessel embolization.

INDICATIONS (Countries that recognize CE marking) – The IMPEDE-FX RapidFill Device is indicated to obstruct or reduce the rate of blood flow in the peripheral vasculature.
Market Approvals: The IMPEDE-FX RapidFill Device has received CE mark. IMPEDE-FX RapidFill is not available for sale in the United States or Japan. Find your local distributor.
Indications, contraindications, warnings, and instructions for use can be found in the product labeling supplied with each device. See all Indications, Safety & Warnings
IMPEDE PRODUCT FAMILY
IMPEDE-FX RapidFill
IMPEDE-FX RapidFill enables the delivery of 6.25 mL of smart polymer in a single application. The device consists of five IMPEDE-FX 12 mm Embolization Plugs preloaded into a single delivery introducer (cartridge), which may provide an advantage in cases where the goal is to quickly fill and occlude large vessels or lesions with reduced procedure and fluoroscopy time, contrast media, and blood loss.
Space Fill with IMDEDE-FX RapidFill is a technique using multiple embolization plugs to fill and occlude large blood vessels or large spaces such as aneurysms or hemorrhage.
Track Fill with IMDEDE-FX RapidFill enables embolization along the vessel of lesion length, a technique as performed for venous insufficiency, pelvic congestion syndrome, and collateral vessel embolization.

INDICATIONS (Countries that recognize CE marking) – The IMPEDE-FX RapidFill Device is indicated to obstruct or reduce the rate of blood flow in the peripheral vasculature.
Market Approvals: The IMPEDE-FX RapidFill Device has received CE mark. IMPEDE-FX RapidFill is not available for sale in the United States or Japan. Find your local distributor.
Indications, contraindications, warnings, and instructions for use can be found in the product labeling supplied with each device. See all Indications, Safety & Warnings