A Smart Approach:
Future Solutions
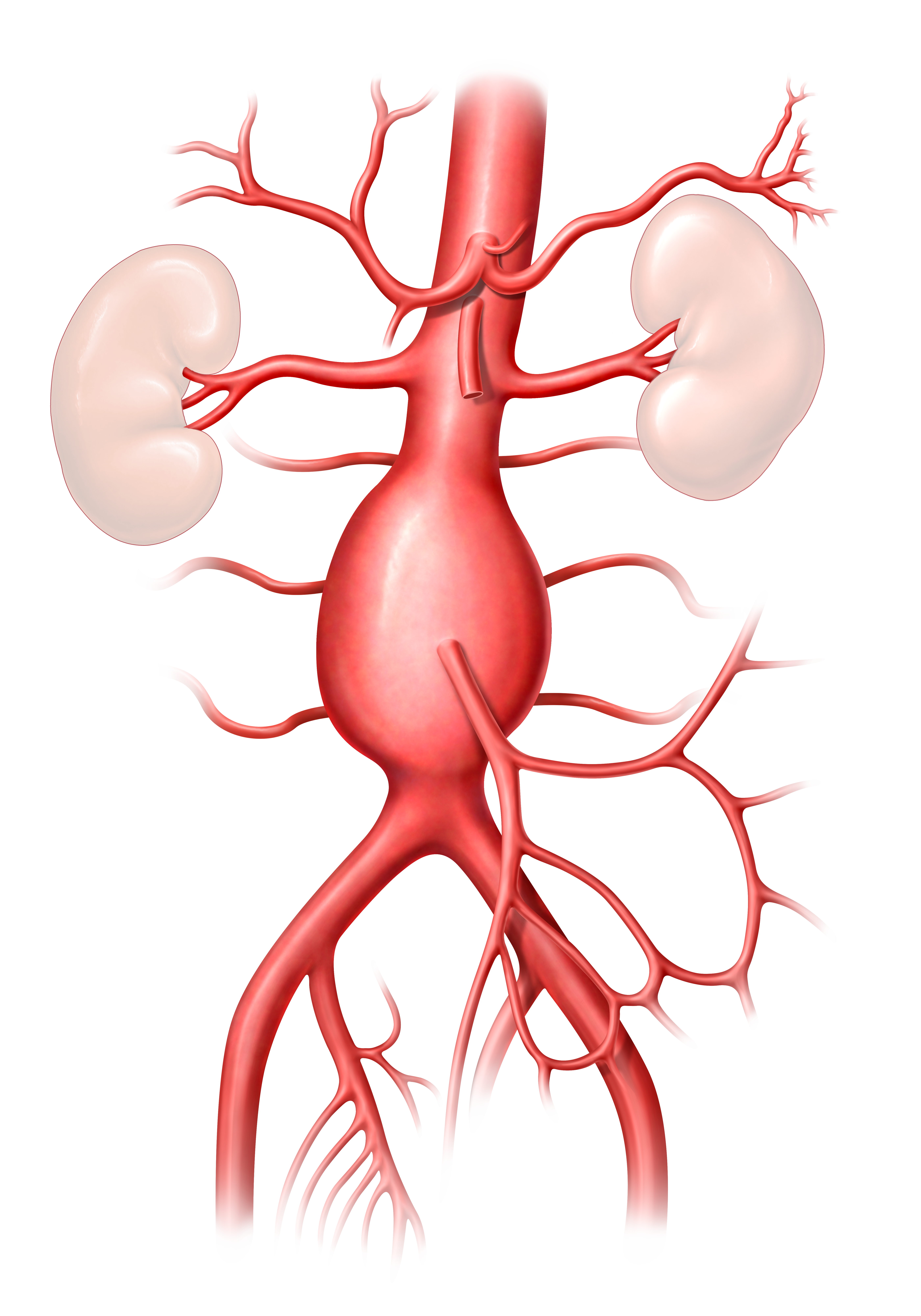
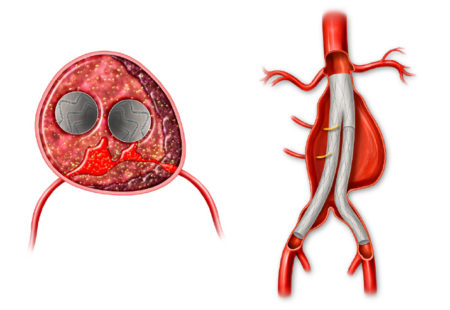
Post-EVAR aneurysm failure to regress remains a significant unmet clinical need associated with higher long-term all-cause mortality.
Shape Memory Medical is committed to evaluating the potential of shape memory polymer to influence AAA sac behavior through various studies.
AAA-SHAPE Pivotal Trial
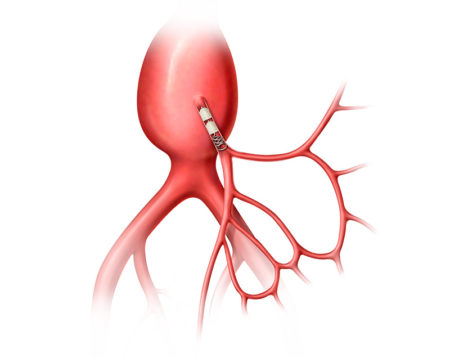
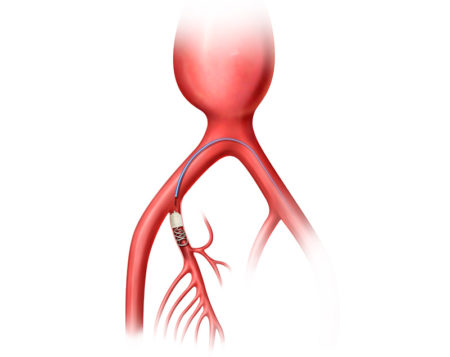
The AAA-SHAPE Pivotal Trial is a prospective, multicenter, randomized, open-label trial to determine safety and effectiveness of the IMPEDE-FX RapidFill Device to improve abdominal aortic aneurysm (AAA) sac behavior with used with elective endovascular aneurysm repair (EVAR).
The trial will enroll 180 patients with infrarenal AAA across 50 sites in the U.S., Europe, and New Zealand. Study participants will be randomized 2:1; either to EVAR plus sac management with the IMPEDE-FX RapidFill (the treatment arm) or to standard EVAR(the control arm). Key endpoints will compare sac diameter and volume change, endoleak rates, secondary interventions, and mortality through five years.

Abdominal Aortic Aneurysm Sac Healing and Prevention of Expansion (AAA-SHAPE)
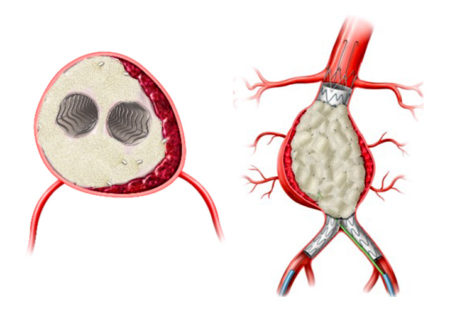
The AAA-SHAPE Pivotal Trial is intended to determine the safety and effectiveness of IMPEDE-FX RapidFill to increase the percentage of subjects with shrinkage of the abdominal aortic aneurysm sac when used as an adjunct to on-label endovascular aneurysm repair (EVAR) stent graft treatment in trial subjects considered candidates for elective EVAR.
AAA-SHAPE Safety and Early Feasibility Studies
AAA-SHAPE FIH is the first prospective clinical trial to evaluate IMPEDE-FX RapidFill in AAA patients and its potential to remodel the AAA sac and improve aneurysm sac regression following EVAR. The study has enrolled 35 patients across three centers in the Netherlands, and two centers in New Zealand.
One year follow-up was completed in 2023.

Abdominal Aortic Aneurysm Sac Healing and Prevention of Endoleaks (AAA-SHAPE)
AAA-SHAPE NZ and NLD are prospective, multicenter safety and early feasibility trials to determine the safety and efficacy of the IMPEDE-FX Embolization Plug and/or IMPEDE-FX RapidFill to fill an abdominal aortic aneurysm (AAA) sac outside of an endovascular aneurysm repair (EVAR) stent graft.